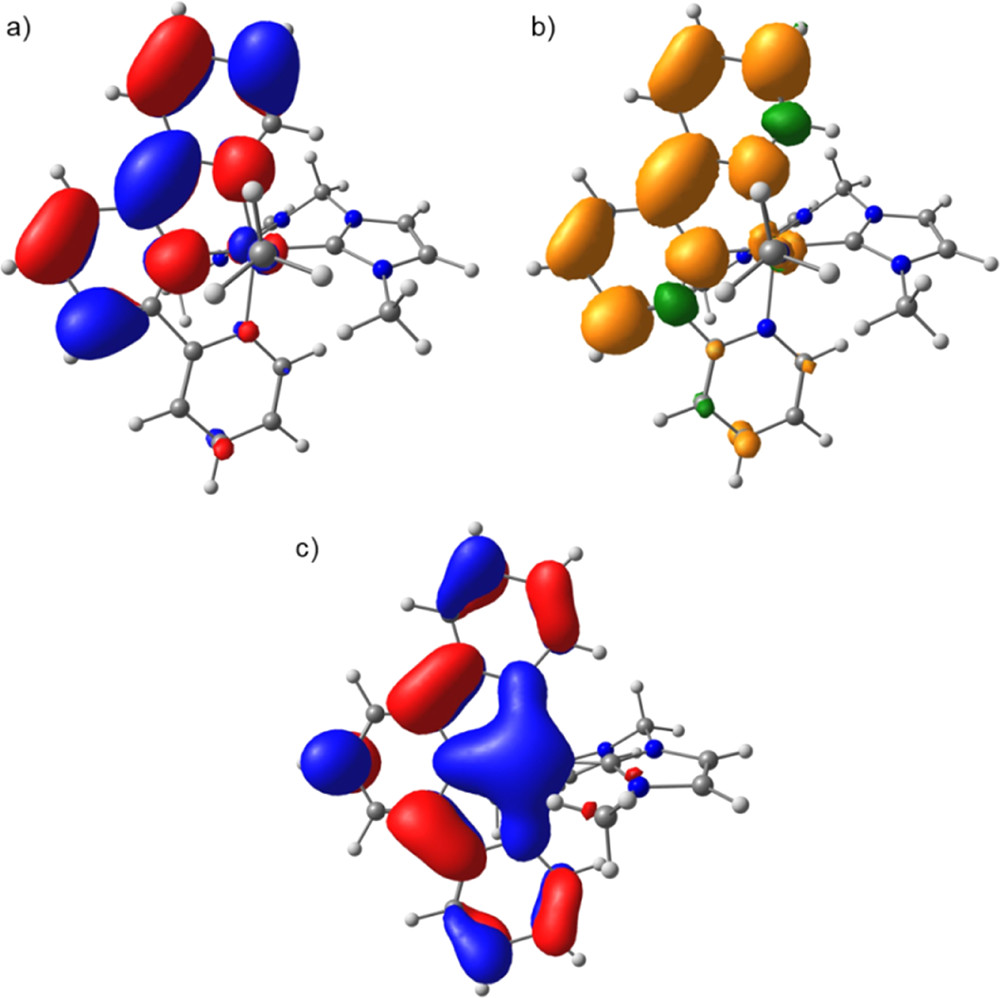
 A common challenge in molecular electrocatalysis is the relationship between maximum activity and the overpotential required to reach that rate, with faster catalysts incurring higher overpotentials. This work follows a strategy based on independent tuning of ligands in the primary coordination sphere to discover a previously unreported iron catalyst for CO2 reduction with higher activity than similar complexes while maintaining the same overpotential. Iron complexes bearing a bis-N-heterocyclic carbene ligand (methylenebis(N-methylimidazol-2-ylidene), bis-mim) and a redox active 2,2′:6′,2″-terpyridine (tpy) ligand were synthesized and found to catalyze the selective reduction of CO2 to CO at low overpotential with water as the proton source. Mechanistic studies based on kinetic zone diagrams, spectroscopy, and computation enable comparisons with a previously studied pyridyl–carbene analogue. Changing the bidentate ligand donor ability accelerates catalysis at the same overpotential and changes the nature of the turnover-limiting step of the reaction.
A common challenge in molecular electrocatalysis is the relationship between maximum activity and the overpotential required to reach that rate, with faster catalysts incurring higher overpotentials. This work follows a strategy based on independent tuning of ligands in the primary coordination sphere to discover a previously unreported iron catalyst for CO2 reduction with higher activity than similar complexes while maintaining the same overpotential. Iron complexes bearing a bis-N-heterocyclic carbene ligand (methylenebis(N-methylimidazol-2-ylidene), bis-mim) and a redox active 2,2′:6′,2″-terpyridine (tpy) ligand were synthesized and found to catalyze the selective reduction of CO2 to CO at low overpotential with water as the proton source. Mechanistic studies based on kinetic zone diagrams, spectroscopy, and computation enable comparisons with a previously studied pyridyl–carbene analogue. Changing the bidentate ligand donor ability accelerates catalysis at the same overpotential and changes the nature of the turnover-limiting step of the reaction.
Gonell, S.; Assaf, E. A.; Lloret-Fillol, J.; Miller, A. J. M. An Iron Bis(carbene) Catalyst for Low Overpotential CO2 Electroreduction to CO: Mechanistic Insights from Kinetic Zone Diagrams, Spectroscopy, and Theory. ACS Catal. 2021, 11, 15212-15222. https://doi.org/10.1021/acscatal.1c04414