About
The Alliance for Molecular PhotoElectrode Design for Solar Fuels (AMPED) is an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science, Office of Basic Energy Sciences
MISSION
To Develop the Fundamental Molecular Basis for Solar-Driven Water Oxidation and Carbon Dioxide Reduction Catalysis
A MOLECULAR APPROACH TO SOLAR FUELS
Sunlight is the one renewable natural resource that could by itself meet all the energy demands of our growing world economy. For solar energy to reach its full potential it must be coupled to a storage method, which can be achieved by conversion of sunlight into chemical energy stored in bonds of molecules called solar fuels. Inspired by natural photosynthesis, the mission of the Alliance for Molecular PhotoElectrode Design for Solar Fuels (AMPED) is to develop the fundamental molecular basis for solar-driven water oxidation and carbon dioxide reduction catalysis. This mission will be achieved by understanding how molecular catalysts and chromophores can be integrated covalently and non-covalently with oxide materials to achieve structurally well-defined photoelectrodes that efficiently couple light absorption with multi-electron fuel-forming reactions. Two integrated research goals support the AMPED EFRC mission and specifically address pressing knowledge gaps in energy science:
- Interface Highly Conductive Oxides with Molecular Light-harvesting Dyes and Photocatalysts.
A new approach to highly efficient light-driven catalysis using simple conductive oxides offers a promising alternative to the prevailing use of insulating or semiconducting oxides. Fundamental studies will elucidate dye-sensitized electron transfer reaction mechanisms and guide the development of new photoelectrode designs. Highly doped transparent conductive oxide (TCO) nanocrystalline mesoporous thin films will be utilized for dye-sensitized solar fuel generation. Recent AMPED EFRC research has demonstrated that, with careful design of the molecule-TCO interface, productive electron transfer reactivity can be realized that enables photocatalysis. In such studies the dye excited state undergoes rapid electron transfer with a molecular donor or acceptor — thereby precluding deactivation by the conductor. With an applied potential, the TCO has been shown to fulfill the role of an n– or p-type semiconductor material by collection of reducing or oxidizing equivalents. This breakthrough discovery changes the way we think about materials, with an emphasis on the position of the Fermi level, EF, of the oxide material under solar irradiance conditions (whereas the band edge position is the key parameter for semiconductors).
Detailed experiments will enable molecular control of interfacial dynamics and catalysis at conductive oxides by determining reorganization energies and reduction potentials associated with electron transfer. Preliminary results have demonstrated both remarkably long-lived charge separation and multi-electron/multi-proton catalysis.
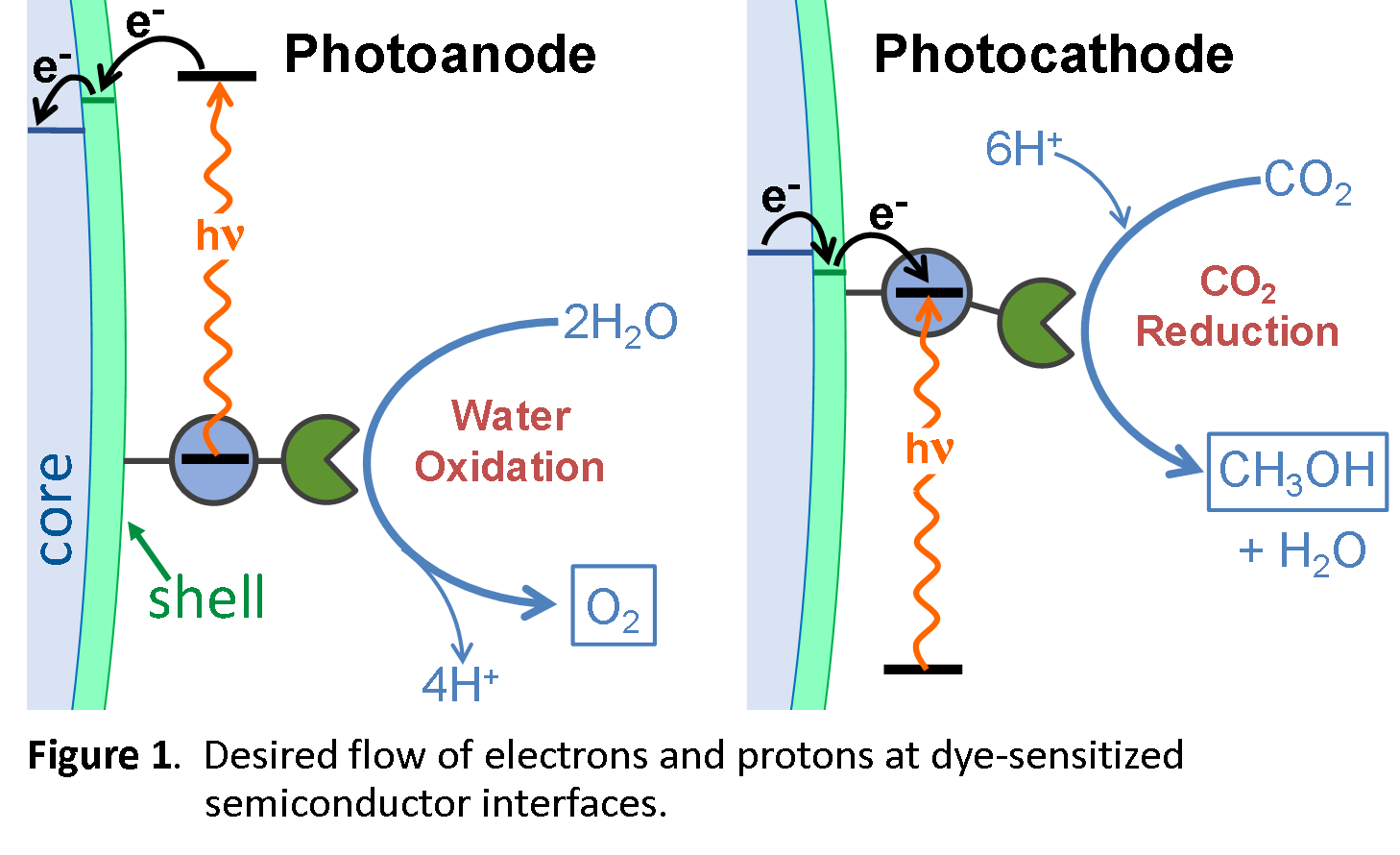
Synthetic photoelectrochemistry for solar fuel production requires that light absorption be efficiently coupled with chemical bond formation, achieved through the directed flow of electrons and protons away from water oxidation catalysts and towards CO2 reduction catalysts, Figure 1. Electron flow has been optimized in prior AMPED EFRC research through the spatial arrangement of redox active centers or band edge positions that provide a free energy gradient. Methods for directional transfer of both electrons and protons do not exist, yet are critically important at oxide interfaces. Interfaces will be designed to direct proton and electron flow both between interfaces and also laterally within a component layer. Unlike the TCOs in Goal 1, catalysis generally occurs at potentials within the forbidden energy gap, conditions where the oxide acts as an insulator and blocks electronic communication with the external circuit. The semiconducting oxides will be exploited to characterize the redox behavior of site-isolated catalysts so as to control intermolecular reactions. The interfacial architectures will be quantified in aqueous solution using time-resolved spectroscopic and electrochemical techniques. By developing synthetic methods that provide spatial control of molecule-material interfaces, and tuning electronic and protonic coupling at interfaces, we aim to identify and understand the basic principles that direct flow between dyes, catalysts, and semiconductor oxides in the dark and under solar illumination.
An approach rooted in comprehensive mechanistic analysis, at the molecular level and at molecule-materials interfaces, will establish new paradigms in light-driven fuel synthesis based on exquisite control over photoelectrode structure and dynamics. AMPED EFRC research will leverage expertise in molecular and materials synthesis and characterization, catalysis, time-resolved spectroscopies, kinetics and modeling, and photo-electroanalytical methods. The proposed multi-disciplinary team-based approach has proven to be highly effective in bringing together a broad spectrum of ideas and capabilities necessary for transformational advances in solar fuels research that could not be realized by individual research groups. The impact of AMPED EFRC research will be a deeper mechanistic understanding of sunlight-driven catalytic transformations at molecule-material interfaces, which will in turn fuel the discovery of new breakthroughs in solar energy conversion chemistry.